What is Galvanizing Process & it’s types & importance?
Galvanizing
Galvanizing
is one of the essential processes in the manufacturing of high-quality fencing.
It is the important tool against corrosion. Different type of galvanizing is
used in the organization that manufactures fencing wire or concertina coil:
Hot dip
Galvanizing
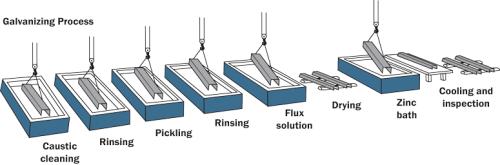
According to
BS EN ISO 1461, hot dip galvanizing is the best way of the coating. In this
process, the element that we want to galvanize is dipped in a bath of hot,
liquid zinc. 419ºC is sufficient to melt zinc, while zinc in molten bath
generally has a temperature between 440 and 460ºC. The galvanized components
are decreased, preserved & fluxed (coated with an adhesive liner) after
that they are dipped in the molten zinc bath then the insides, welds & hard
to reach corners are well coated.
The minimum
layer of thickness for the objects utilized outside as indicated by BS EN ISO
1461, contingent upon the thickness of the steel, is 70 µm or over. Along these
lines of Zinc covering offers most extreme security from corrosion, yet is
exceptionally relentless. Because of warmth included, joints can pull loose or
the material can twist. If there should arise an occurrence of the hole like in
empty areas, dipping must be done deliberately, as to not give the trapped air
a chance to extend and make the object detonate. Furthermore objects regularly
should be polished and buffed, because the layer of Zinc is thick and uneven.
Pre-Galvanizing:
This type of
galvanizing is frequently utilized as a part of our industry of tubes or empty
segments. In the process the strip steel, straight from the coil is driven
through a preheating stove, pre-treatment showers and the molten Zinc baths at
high speeds before the segments or tubes are moved from it. While leaving the
Zinc bath the abundance Zinc is cleaned off with packed air and blown back to
the bath. Thusly the thickness of the layer can be resolved reasonably
correctly, generally, it is between 15 and 20 µm. The last station in the
process makes the surface much smoother than the hot dip galvanizing does,
while the hold of the Zinc on the steel is similarly as great. The weakness
then again is, on the grounds that the tubes and segments are rolled and hence
welded after the Zinc plating, the welds are not generally all around secured
against corrosion. Although by welding the Zinc on the two sides of the welding
line warms up, melts again and mostly flows into the weld, this is insufficient
to totally secure the weld from corrosion. Some rolling factories consequently
provide a subsequent treatment with a cold Zinc coating, however even along
this way of Zinc coating is successful just when utilized on materials that are
powder coated after being galvanized. Since both follow up the action with cold
galvanizing coating and powder coating are just conceivable outwardly of the
area, the weld on the inside of the section is protected from corrosion only
minimally.
Centrifugal
Galvanizing:
It is the
third way of zinc coating, used for small parts which are very not easy to hang
up or have little gaps that would fill up with Zinc in standard hot dip
galvanizing. In this galvanizing, the parts are kept in a container. This
container is then dipped in a bath of fluid Zinc. The temperature of this bath
is generally 530ºC, so the Zinc is more slender. By quickly pivoting the crate
when it leaves the bath, the parts are centrifuged, in this way lessening the
layer thickness. By altering the time of centrifuging the layer thickness can
be balanced absolutely.
Electroplating:
Electroplating
or electrolytic galvanizing is the process that comprises of fumigation of
metal particles on another metal. It's precisely the same that is utilized for
chromium-plating or nickel-plating objects. The strategy functions as follow –
Zinc chloride is melted in a bath of water. It breaks down in Zn2 cations and
Cl anions. The object to be Zinc plated is hooked up to the negative pole of a
present source, in this manner turning into the cathode. Another metal object
is hooked up to the positive pole, turning into the anode. When the two
articles are submerged into the Zinc chloride bath, an electric current will
flow from positive to negative. The Zinc particles now retain electrons and
disinfect on the cathode (the object to be galvanized) as Zinc. Just the chlorine
solution stays in the bath. The big advantage of electroplating is that you
require the least amount of Zinc (eventually a layer is just 7 or 8 µm) to make
an exceptionally smooth surface that perfectly seals in the steel, hence
shielding it from corrosion. Hence the layer is so thin, it is sensitive to
harming.
If you are
interested to buy security wire coils products kindly visit http://www.saiwire.com
Post Your Ad Here
Comments