Client Success Story: Over €200 Million! Congratulations to I-Mab Biopharma
Client Success Story: Over €200 Million! Congratulations to I-Mab Biopharma for Successful Licensing to Sanofi — SHUIMU BIO Powers New Collaboration on CD73 Target Drugs
On September 25, 2024, SHUIMU BIO received exciting news from our client, I-Mab Biopharma: their innovative CD73-targeted cancer therapy has been successfully licensed to Sanofi, with a total deal value exceeding €200 million!
I-Mab Biopharma, a leader in innovative biologics research and development, recently announced a strategic collaboration with global pharmaceutical giant Sanofi. Under this agreement, I-Mab licensed the development, production, and commercialization rights for their proprietary CD73 antibody, uliledlimab, in Greater China to Sanofi. I-Mab is set to receive approximately €32 million in upfront and near-term milestone payments, with a potential total consideration of up to €213 million (approximately 1.7 billion RMB).
The Importance of CD73 Target in Cancer Immunotherapy
The CD73 target plays a crucial role in the tumor micro-environment. By catalyzing the conversion of AMP to adenosine, it creates an immunosuppressive environment that promotes tumor growth. Uliledlimab effectively inhibits CD73 activity by a unique binding mechanism, reducing adenosine levels and enhancing the activity of anti-tumor immune cells, demonstrating significant potential for cancer therapy. The research and development of CD73-targeted drugs have become a new hotspot in cancer immunotherapy.
SHUIMU BIO’s Contribution to CD73 Antibody Development
In 2021, SHUIMU BIO assisted I-Mab Biopharma with the structural analysis of the CD73 antibody, providing strong scientific support for the clinical translation of this innovative drug. Utilizing cryo-electron홓흏 microscopy (Cryo-EM) single particle analysis, SHUIMU BIO successfully resolved the 3D structure of the CD73 dimer-antibody complex, revealing the non-competitive mechanism of action and visually highlighting the differences between uliledlimab and similar antibodies. This achievement provided fundamental evidence for I-Mab's clinical trials.
Case Review
The previously mentioned antibodies either need to be in full-length form to inhibit enzyme activity, or they have poor efficacy as monoclonal antibodies (mAbs), requiring modification into bispecific antibodies to enhance potency. However, in this study, the mAb demonstrated equivalent efficacy even with single Fab binding, without depending on the Y-shaped structure of the antibody. This suggests that its mechanism of action may differ from those of the previously discussed antibodies.
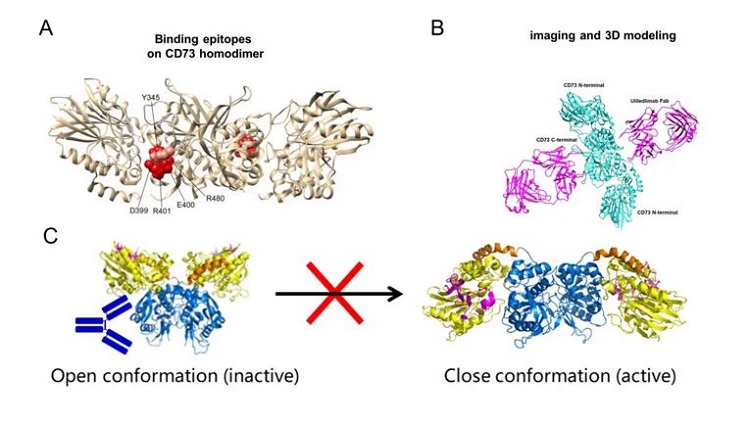
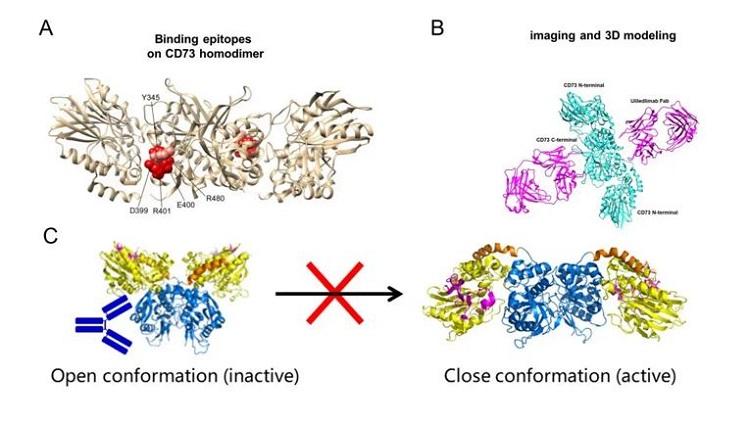
As the only antibody currently known to bind to the C-terminal domain of the CD73 protein, its binding site is unique, located at the periphery of the dimer interface of the C-terminal domain. Although this site is close to the substrate binding pocket, it does not interfere with the substrate-CD73 interaction (Figure 2.A). In epitope analysis, we first consider the relationship between the antibody binding site and the substrate pocket. The most common antibody mechanism is competitive inhibition with the substrate, which requires a high level of antibody-antigen affinity. Additionally, the diversity of substrates makes antibody selection more challenging. More importantly, the active sites of enzymes often share high sequence homology, increasing the risk of off-target effects in drug development. Therefore, non-competitive antibodies that bind through allosteric mechanisms, locking the protein in an inactive conformation to inhibit enzymatic activity, are an important approach in antibody drug development.
Comparing the structure of the CD73 dimer bound to the Fab antibody with the Open and Closed states of the CD73 dimer, we observe that the structure resolved in this study lies between the Open and Closed conformations. The antibody creates significant steric hindrance with the N-terminal domain of the protein in the Closed active state, preventing the substrate and ion binding regions from approaching each other. This allosteric inhibition locks CD73 in its inactive form, and the binding of a single Fab is sufficient to exert its effects. The ratio of CD73 to antibody in the system does not significantly affect the inhibitory effect of the antibody. Furthermore, the antibody’s binding at the periphery of the C-terminal domain allows for a wider range of binding angles and a higher likelihood of successful binding. Structural studies have effectively validated and explained in vitro results, such as affinity, enzymatic activity, and the hook effect.